Securecell AG/Vetsuisse-Fakultät ZH, MSRU/CABMM, May and November 2022
Keywords: Seraccess, automated blood sampling, animal model
Background
Automated blood glucose measure-ment with Securecell’s SeraMaster prototype was tested in a clinical setting as part of a feasibility study (see Milestone 6).
Several experiments on live sheep were defined to test automated blood sampling in vivo. In a first se-ries of experiments, the automated sampling process, including dilution with NaCl, was compared with man-ual sampling. In a second series of experiments, automated blood sam-pling was combined with automated glucose measurement.
Study design
The trial (national authorization number: 34196, cantonal: ZH215/2021) was conducted in col-laboration with the Vetsuisse Faculty of the University of Zurich. It includ-ed seven full-day trials. Blood sam-ples were automatically collected with the SeraMaster prototype via central venous catheter (n=128) or arterial catheter (n=104) every 15 minutes. They were then compared with the manually collected refer-ence samples by measuring the glu-cose concentration of each sample.
Results
To evaluate the automated method, the bias (=mean deviation from the reference method) was calculated. For samples from the central vein the bias was 1.8 mg/dl (n=96) and for samples from the peripheral artery it was 0.8 mg/dl (n=104). All 200 data points (100%) are within the margin of error of ± 10.0 mg/dl.
The following figure shows the re-gression analysis between SeraMaster glucose values (y-axis) and manual sampling (x-axis). The correlation coefficient (R2) is 0.9930, which means that the two methods are equivalent and therefore inter-changeable.
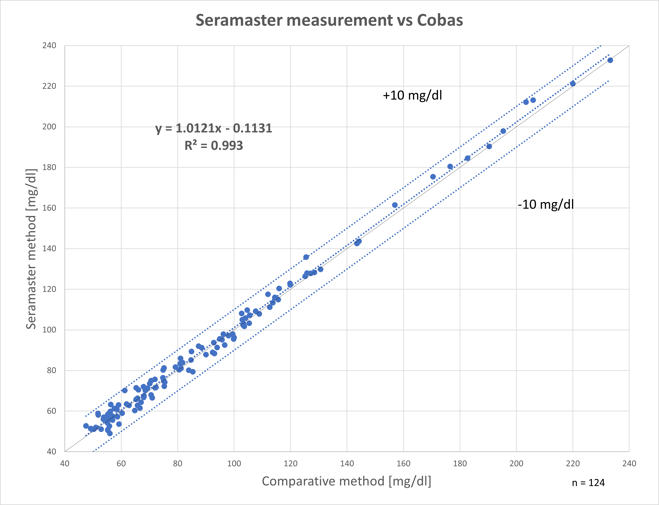
The regression analysis between the glucose values of SeraMaster (Y-axis) and of Cobas C111 (X-axis).
Furthermore, it could be shown that a continuously administered glucose infusion in the same two-lumen central venous catheter (proximal lumen: sampling, distal lumen: glucose infusion) had no influence on the automatic glucose measurement with the SeraMaster prototype (n=32).
The images illustrate the experimental setup in the stables of the university. At the end of the day, the laboratory animals were allowed to return to the pasture.

Next steps
Next, automated blood sampling will be tested in a clinical trial.
| Continuous automated blood sampling combined with automated glucose measurement with the SeraMaster prototype is equivalent to the manual reference method. The bias, a measure of the accu-racy of the method, is 1.8 mg/dl (venous) and 0.8 mg/dl (arterial) compared to the reference method. The regression analysis shows that the two methods are interchangeable. |










