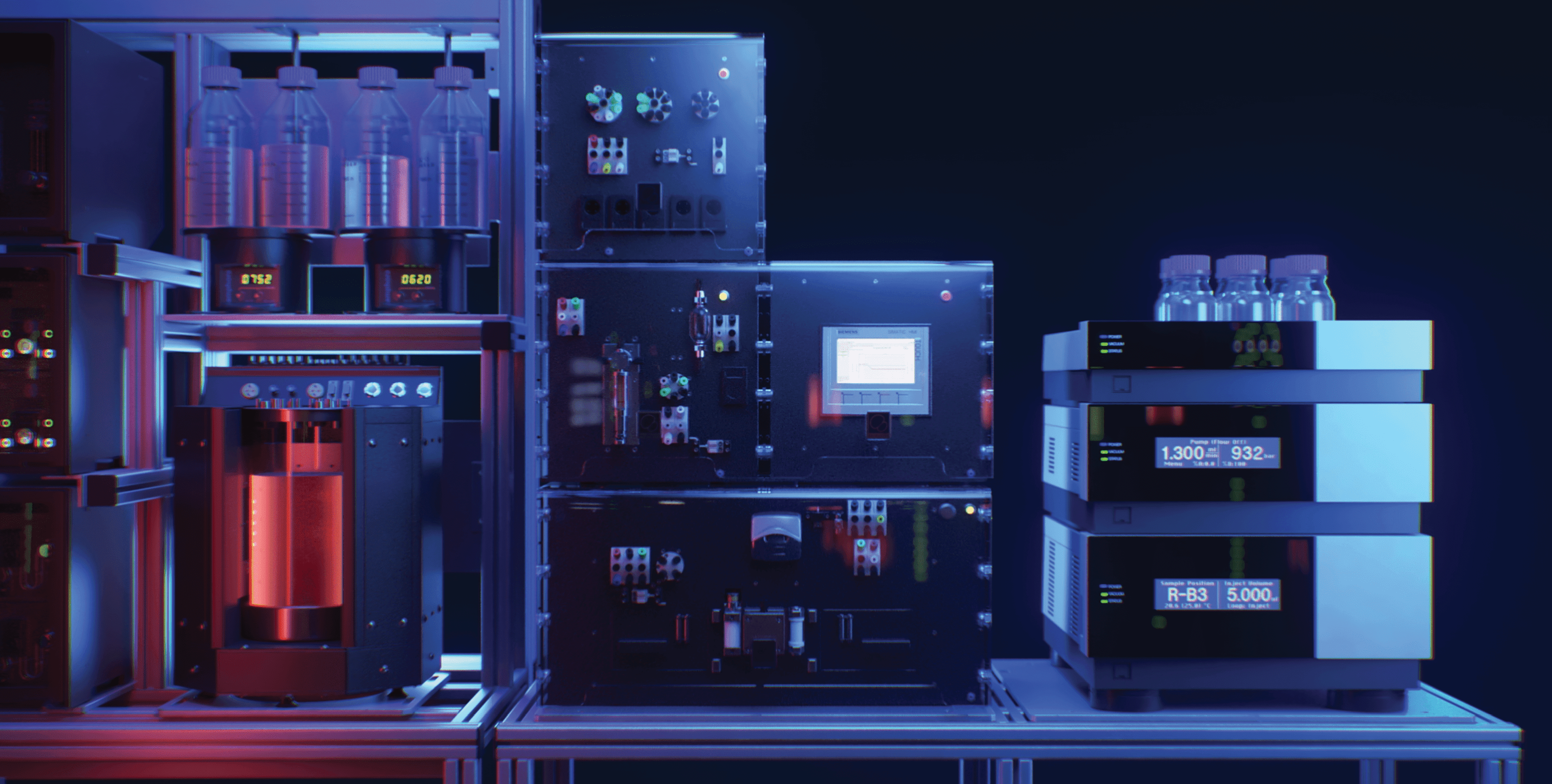
A recently published article in the Journal of Chromatography A (Volume 1672, 7 June 2022, 463067; available online from April 15, 2022) shows that on-line monitoring of quality attributes (QAs) directly within the bioreactor can provide the basis for advanced modes of protein production including process intensification, smart manufacturing, and real-time release testing.
The presented method demonstrated for the first time an on-line peptide mapping platform in upstream cell culture production with integration and combination of an automated sampling system and a multi-dimensional (mD)-LC-MS analyzer. The platform was used to automatically draw a sample from the bioreactor with the Numera® sampling system (Securecell AG), seamless sample transfer to a liquid chromatography system, chromatographic purification, followed by charge variant and peptide mapping analysis. This technology enables the near-real-time monitoring of numerous post-translational modifications during the cell culture process, including oxidation, succinimidation, sialylation, deamidation, and glycosylation, for both an IgG1 and an Fc-fusion protein. The described approach is fully automated from sample drawing to data generation and requires no further manual involvement.
The automated sampling system Numera® was operated using the Lucullus® process information management software (Securecell AG), which allows samples to be drawn at preset intervals or scheduled times. Within Lucullus®, critical information to inform downstream analyzer operations could be input (sequence name, instrument method, and processing method). After storing the sample in the liquid handler, sequence information and analysis parameters were transmitted automatically to Chromeleon to enable automated method initiation. A software adapter developed and provided by Securecell AG enabled communication between Lucullus® and the software of the mD-LC system (Chromeleon 7.3 from Thermo Scientific™) to control downstream processing/analysis.
Key benefits of the on-line availability of the mD-LC-MS system are the possibility to integrate feed-back loops, more consistent product quality, reduced burden on downstream unit operations to remove impurities, and reduced effort for extensive process characterization.
Original article written by Sanket Dahotre; Lu Dai; Karissa Kjenstad; Cinzia Stella; Julien Camperi
Read the full publication on Journal of Chromatography A Volume 1672, 7 June 2022