Biopharma Manufacturing Challenges
Biopharmaceutical laboratories and production plants typically rely on work procedures involving many manual handling steps, ranging from material preparation to sample analysis. Large equipment fleets from different vendors with stand-alone configurations, different data formats, and communication protocols make the implementation of modern Industrial Internet of Things (IIoT) or Industry 4.0 production concepts challenging. Moreover, regulatory constraints disfavor changes in existing production setups or processes after validation and approval.[1]
Innosuisse Project Goals
The development of the technical framework for bioprocess intensification through digitalization and automation concepts was the goal of the Innosuisse-supported project between Securecell AG and the Bioprocess Research Group at ZHAW. The potential of unified and automated monitoring, control, and evaluation routines across a wide range of biotechnological value chains (from bacteria to mammalian and microalgae cultures), was explored.[2]
The project included the following focus areas:
-
Expansion of the existing Lucullus installation with the connection of a wide range of bioprocess devices
-
Complete digitization of the media and reagents preparation step:
- Digital raw material management
- Digitized protocols with a digitally supported workflow
- Integration of balances, pH meters, and osmometers that automatically transmit measurement values to the digitized protocols
- Assignment of the media to the respective processes using barcodes
-
Extension of the Numera® sampling system with a module for pH measurement
-
Indirect integration of spectral data (RAMAN) in Lucullus®
-
Development of algorithms for data evaluation, process monitoring, and control
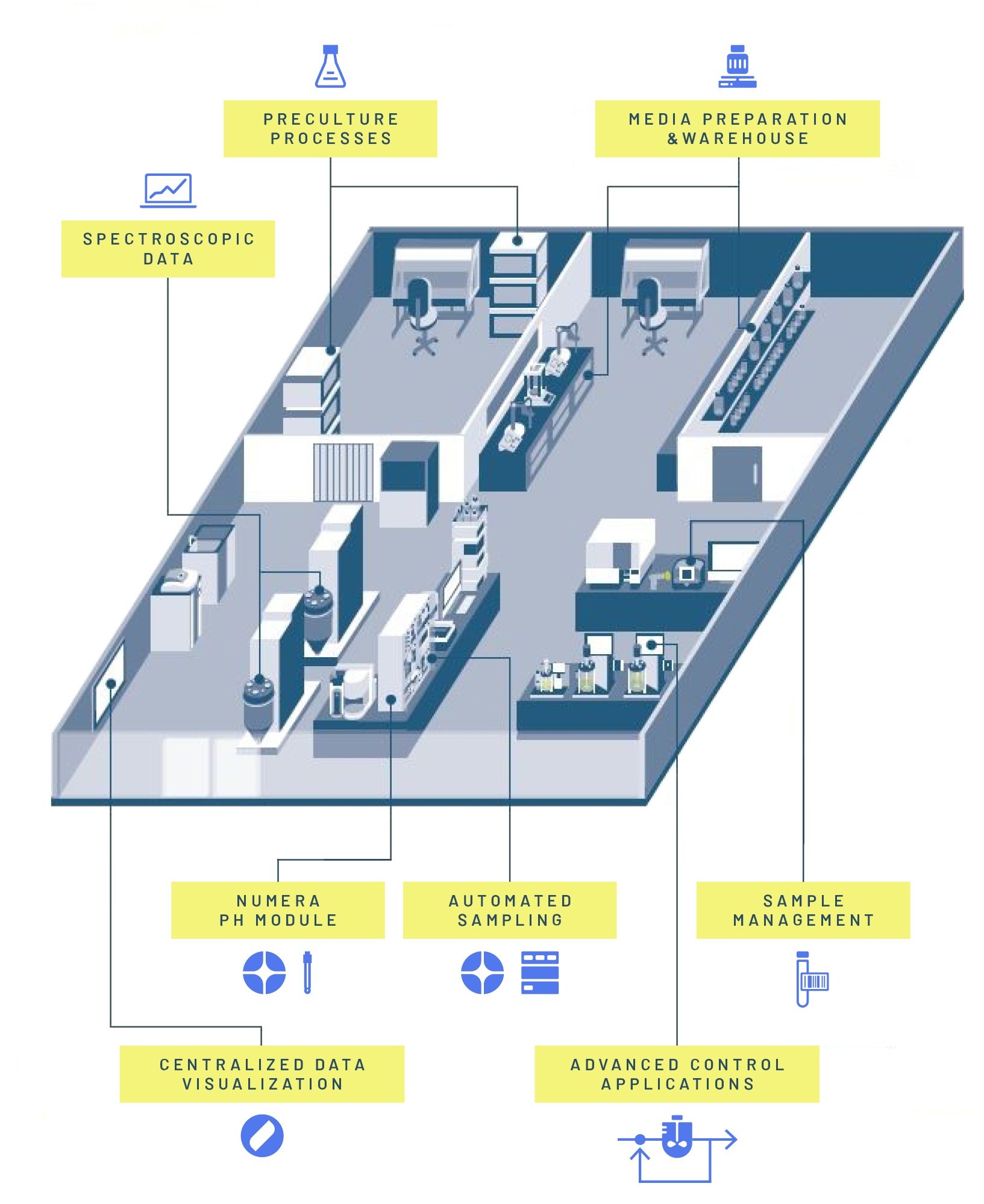
The i2BP Lab (Intelligent and Integrated BioProcessing Lab)
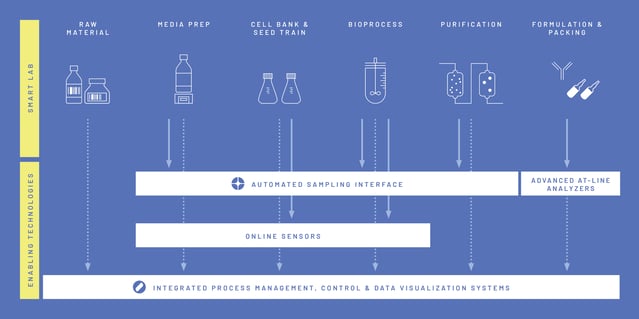
The main output of the project was the planning, construction, and creation of a fully integrated process development and manufacturing ecosystem with digitally enhanced workflows called i2BPLab (Intelligent and Integrated BioProcessing Lab). By bridging gaps between traditionally isolated work units, a holistic view of the entire processing chain was enabled, opening the door to "advanced" methods of process development and data exploitation.[3]
The bioprocess laboratory at the Grüental Campus at the ZHAW in Wädenswil has a dedicated laboratory infrastructure that links the existing hardware and software from different manufacturers in the best possible way. At the heart of bioprocess digitization is the comprehensive Lucullus® software solution from Securecell AG, which allows process chains to be digitalized from media production through precultures and bioreactors to algorithm-based data evaluation. Supported by automated sampling and analysis with the Numera® sampling system, also from Securecell AG, bioprocesses can be largely automated and thus developed and executed with maximum efficiency.
In the future, both students and research groups at the ZHAW will benefit from the unique digital technologies. The high-quality equipment of the laboratory also opens up new possibilities for Securecell AG. Products can now be tested extensively and efficiently in real applications, improved in collaboration with the ZHAW, and demonstrated to potential customers.
A small part of the project budget was used to showcase the modern laboratory to the ZHAW students and to industry partners to get an insight and explore the available equipment and facilities.
Discover the i2BP lab in a virtual tour now!
https://my.matterport.com/show/?m=rRypssMvxt4
 References
References
[1] Olivier Vorlet, Lukas Neutsch, Christian Kronseder, and Alexandre Kuhn, Digitalization in Processes, Chimia 75 (2021) 1–8[2] C. Herwig, O. F. Garcia-Aponte, A. Golabgir, A. S. Rathore, Trends Biotechnol. 2015, 33, 381, https://doi.org/10.1016/j.tibtech.2015.04.004.
[3] L. Neutsch, Eur. Biopharm. Rev. 2021, 2021, 57.




