Article
LUCULLUS® AS THE CONTROL SOFTWARE IN A NOVEL METHOD FOR ANAEROBIC SINGLE-CELL PROTEIN PRODUCTION
November 18, 2025Innovative anaerobic process using Lucullus® software optimizes single-cell protein production, achieving high cell densities and protein content, minimizing by-products in submerged cultivations.
PRECISION IN EVERY DROP: PERFECT GRAVIMETRIC FEEDING WITH LUCULLUS® SOFTWARE
September 15, 2025Discover how Lucullus® software optimizes gravimetric feeding for bioreactors, ensuring precision in medium supply and enhancing biotechnological processes at Octarine.
LUCULLUS® RELEASE NOTES VERSION 24.1.2
August 14, 2025Discover the new features and improvements in Lucullus® version 24.1.2, including enhanced usability, extended REST API, and various bug fixes.
PRECISION MONITORING OF OBLIGATE ANAEROBES DURING FERMENTATION
July 16, 2025Precision monitoring of anaerobes during fermentation using Securecell's Numera® system ensures accurate viability assessments and minimal oxygen exposure for pharmaceutical development.
ADVANCED PROCESS MONITORING: CELL DENSITY AND METABOLITE ANALYSIS IN ADENOVIRUS PRODUCTION WITH LUCULLUS®
June 12, 2025Advanced monitoring methods enhance adenovirus production by utilizing Lucullus® software for real-time cell density and metabolite analysis
NAVIGATING FOR THE IDEAL BIOPROCESSING SOFTWARE SOLUTION
September 10, 2024Learn the benefits of using one overarching software solution that accommodates the creation of process control operations/recipes for various environments.
LUCULLUS® RELEASE NOTES VERSION 24.1.1
July 23, 2024Securecell AG has released a new version of Lucullus®. The latest update is 24.1.1 based on the new version naming convention.
AUTOMATED EVOLUTIONARY ENGINEERING OF MICROORGANISMS IN BIOREACTOR SYSTEMS USING THE SOFTWARE LUCULLUS®
March 14, 2024The article explores how automating evolutionary engineering with the Lucullus® system can significantly enhance the efficiency of bioethanol production.
ADVANCED BIOPROCESS MONITORING AND CONTROL VIA THE LUCULLUS® REST API INTERFACE
February 28, 2024The Lucullus® REST API interface meets the requirements to offer a seamless data exchange capability within a local network or over the Internet.
VIRTUAL TOUR OF THE DIGITIZED BIOPROCESSING LABORATORY AT THE ZHAW
February 22, 2024Securecell AG and the Zurich University of Applied Sciences (ZHAW) have successfully completed a joint research collaboration project supported by Innosuisse - the Swiss Agency for Innovation Promotion.
LUCULLUS® RELEASE NOTES VERSION 24.1.0
January 25, 2024Securecell AG has released a new version of Lucullus®. The latest update follows version 23.0.0 and is named version 24.1.0 based on the new version naming convention.
GETTING A GRIP ON OXYGEN TRANSFER IN BIOREACTORS: AUTOMATED kLa DETERMINATION USING THE SOFTWARE LUCULLUS®
January 8, 2024This application note outlines a fully automated method to determine the kLa parameter based on the static gassing-out method, using the software Lucullus® and Getinge Applikon bioreactor systems.
LUCULLUS® AS CENTRAL PROCESS CONTROL SOFTWARE IN GMP PRODUCTION OF MONOCLONAL ANTIBODIES
June 28, 2023In this success story, we provide an overview of the application of our products at one of our major customers from process development to GMP production using the example of a mAb process .
LUCULLUS® RELEASE NOTES VERSION 23
April 13, 2023Securecell has released a new version of Lucullus®. The latest update comes with many new features such as UTF-8, Windows 11 and new Linux OS Support.
ADVANCED REAL-TIME MONITORING IN A CONTINUOUS BIOPROCESS
March 21, 2023Automated sampling with Numera® as a central hub along a continuous bioprocess, enabling advanced real-time monitoring and process wide-control.
DIGITALIZATION AND STATE-OF-ROUTINE BIOPROCESS MONITORING AND CONTROL
March 21, 2023This article introduces the Lucullus® installation at Teva and highlights the workflow improvements through centralized process and data management.
SERACCESS® MILESTONE 7B
February 6, 2023Continuous automated blood sampling including glucose measurement in an animal model
SERACCESS® MILESTONE 7A
January 7, 2023Continuous automated blood sampling based on an animal model
FACILITATED AND RELIABLE PROCESS MONITORING DURING VACCINE PROCESS DEVELOPMENT AND PRODUCTION USING THE AUTOMATED SAMPLING SYSTEM NUMERA®
October 26, 2022Vaccines are one of the most effective, cost-efficient, and sustainable ways of controlling infectious diseases and have had a significant impact on public health and longevity as well as intensive livestock production.
VACCINE MANUFACTURING USING ICELLIS® REACTOR SYSTEMS IS ORCHESTRATED BY LUCULLUS® IN A VALIDATED ENVIRONMENT AT IDT BIOLOGIKA
October 9, 2022The demand for vaccines, cell and gene therapeutics, and generally biologic manufacturing has continuously increased over the past decade.
SEAMLESS CANDIDATE ANTIMICROBIAL AGENT PRODUCTION AT THE HZI BRAUNSCHWEIG SUPPORTED BY LUCULLUS®
September 27, 2022In the “microbial drugs” department at the Helmholtz Center for Infection Research (HZI) in Braunschweig Germany, a high-tech laboratory environment was built embracing the bioprocessing 4.0 cornerstones.
LUCULLUS® MEDIA KITCHEN - DIGITALLY TAILORED MEDIA PREPARATION
August 25, 2022Bioprocessing 4.0, the biopharmaceutical version of Industry 4.0, is defined as the digital transformation and automation of biopharmaceutical processes with the aim to drive manufacturing ...
LEVERAGING SOFTWARE INNOVATIONS FOR AUTOMATED DEVELOPMENT AND OPTIMIZATION OF MAMMALIAN CELL CULTURE BIOPROCESSES
May 16, 2022Bioprocess laboratories with multiple lab-scale bioreactors often execute cultivation experiments in parallel with varying process parameters for process development and optimization purposes.
AUTOMATED ON-LINE CELL CULTURE MONITORING USING NUMERA® AND THE CEDEX® HIRES ANALYZER
May 4, 2022Every biopharmaceutical process aims for real-time monitoring and control of critical process parameters to minimize process variability and at the same time ensure predefined product quality.
NUMERA® ENABLES ON-LINE MD-LC-MS PEPTIDE MAPPING TO MONITOR MULTIPLE POST-TRANSLATIONAL PROTEIN MODIFICATIONS
April 15, 2022A recently published article in the Journal of Chromatography A (Volume 1672, 7 June 2022, 463067; available online from April 15, 2022) shows that on-line monitoring of quality attributes (QAs) directly within the bioreactor can provide ...
LUCULLUS EMPOWERS OVER 100 BIOREACTORS AT AMYRIS
April 12, 2022In 2021 Securecell engaged with Amyris, a biotechnology company headquartered in Emeryville, California and publicly traded under $AMRS on Nasdaq.
RELEASE NOTES LUCULLUS VERSION 3.11
April 4, 2022Securecell AG has released a new version of Lucullus PIMS. This latest update called version 3.11 comes with many new features, focusing on a faster and easier user experience, with many functions brought to a higher level, ...
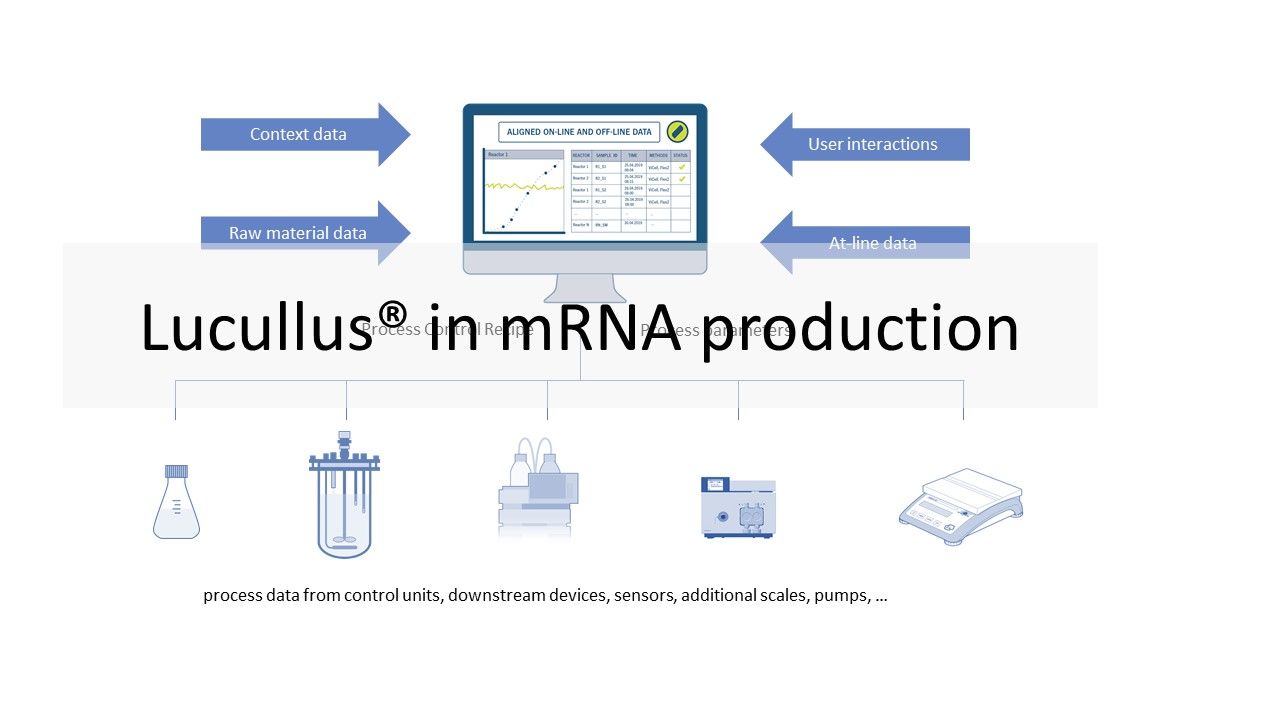
LUCULLUS®: VALIDATED DATA HISTORIAN FOR MRNA VACCINE PRODUCTION PROCESSES
February 10, 2022Vaccines based on messenger RNA (mRNA) gained awareness during the COVID-19 pandemic. This novel alternative to conventional vaccines is emerging for several reasons: on the one hand, mRNA vaccines only ...
SECURECELL PRESENTED SERACCESS AT ATTD CONFERENCE
January 5, 2022SeraMaster Version 2 – Intravenous blood glucose measurement with subjects with type 1 diabetes
COMPARISON OF NONINVASIVE, IN-SITU AND EXTERNAL MONITORING OF MICROBIAL GROWTH IN FED-BATCH CULTIVATIONS IN CORYNEBACTERIUM GLUTAMICUM
March 31, 2021Automated bioprocess systems rely on accurate online measurements of cellular growth. In the present study, six sensors representing five different technologies, namely near-infrared absorbance, ....
BIOPROCESSING 4.0 DEMANDS (AND REWARDS) FINE-TUNED COORDINATION
March 2, 2021Bioprocessing 4.0 is all about digitalization and how it can streamline the analysis and multidirectional communication of data—be it between equipment, among unit operations, or across groups and stages of development.
SERACCESS® MILESTONE 2A
February 3, 2021IV blood sampling for glucose measurement and IV insulin delivery, combined with a closed-loop controller provide effective blood glucose management
SERACCESS® MILESTONE 6
January 11, 2021SeraMaster Version 2 – Intravenous blood glucose measurement with subjects with type 1 diabetes
SERACCESS® MILESTONE 5
January 9, 2021Evaluation of the automatic blood sampling, including sample preparation
SERACCESS® MILESTONE 2B
January 6, 2021Developing a Novel Automated Intravenous Insulin Delivery System. An In Silico Study Characterizing the Glucose Measurement Effect on the System’s Performance
SERACCESS® MILESTONE 4C
January 3, 2021SeraMaster – Intravenous Blood Glucose Measurement; YSI Reference
SERACCESS® MILESTONE 4A
January 3, 2021SeraMaster – Intravenous Blood Glucose Measurement; Cobas C111 reference
NEXT GENERATION MEAT
December 2, 2020The world population is expected to reach 9.6 billion people by 2050, the middle class is growing, and meat consumption is expected to increase by 70%. Meat production requires a great amount of resources: 34 kgs of feed are necessary to produce 1 kg of meat, the same kg of meat requires ca..
PAT SOLUTIONS FOR THE FUTURE OF BIOPROCESSING 4.0
November 26, 2020Pharmaceuticals have a prominent role in healthcare and their manufacturing requires innovative tools, scientific and engineering knowledge, and must at the same time comply with strict regulations.
AUTOMATION INCREASES PRODUCTIVITY IN BIOPROCESS DEVELOPMENT
October 14, 2020The design space of bioprocesses strongly depends on the technological limits of a system. Digitalization helps to push these boundaries and reach new optimization. A vivid example of this technical extension is the use of additional PAT tools to monitor and control bioprocesses.
EMPOWERING BIOPHARMA FOR FULL BIOPROCESS DEVELOPMENT AUTOMATION
August 19, 2020Securecell offers a complete automated 24/7 device for sampling and sample processing to “close the loop” between analytics and process units. Discover in this GEN tech article how Numera® can dramatically implement productivity in biopharma process development.
THE HIGH-TECH SECRET BEHIND THE TRADITIONAL CHEESE SWISSNESS
July 2, 2020Cheese is Switzerland’s most important agricultural-export product: around 170’000 tons of cheese are produced per year (40% of the total Swiss milk amount) with a value of around CHF 600 million. Agroscope, a federal research institute in Liebefeld, near Bern, Switzerland ....
BIOPROCESS INFORMATION MANAGEMENT WITH LUCULLUS SOFTWARE FOR BIOCHEMICAL ENGINEERING AT TU WIEN
April 3, 2020At Technische Universität Wien an innovative laboratory develops and optimizes a wide variety of bioprocesses; those operations require multiple bioreactors and equipment and produce an incredible amount of data.
SERACCESS® MILESTONE 3
January 10, 2020Long-Term Testing of Blood Sampling and Dilution via the In-House Developed Catheter
SERACCESS® MILESTONE 1
November 1, 2019A BodyPort implantation for our automated glucose measuring device for diabetes care was successfully tested in an animal trial.
LUCULLUS SOFTWARE FOR EVENT-BASED PROCESS CONTROL
October 9, 2019Discover how Lucullus PIMS software simplifies advanced bioprocess control through real-time data, exemplified by automated temperature shifts in E. coli processes using optical density and HPLC analysis.
BIOPROCESS SAMPLING: A NECESSARY EVIL OR A CHANCE FOR IMPROVED MONITORING AND CONTROL?
October 9, 2019Monitoring and control of critical process parameters (CPPs) in various bioprocesses is a prerequisite to gain a safe product and hence, is a major topic in Process Analytical Technology (PAT).
A RELIABLE AUTOMATED SAMPLING SYSTEM FOR ON-LINE AND REAL-TIME MONITORING OF CHO CULTURES
October 9, 2019Timely monitoring and control of critical process parameters and product attributes are still the basic tasks in bioprocess development. The current trend of automation and digitization in bioprocess technology targets ...
SIMPLY GOOD MONITORING
September 10, 2019Digitising process development can reduce the time delay between sampling and availability, and can increase the quality of the data obtained.
“STRETCHING THE RANGE” WILL NOT SOLVE THE PROBLEM
June 6, 2019In healthy people, pancreas regulates blood glucose in such a way that it is practically always within the target range. The so-called “time in range” (% of the time in 24 hours during which the blood ...
IN SANGUINE VERITAS!
April 9, 2019In discussions with analysts and potential investors, we occasionally hear the argument that there is no relevant need for the Seraccess Diabetes Care project, “because Novartis (Alcon) and Google (Verily) are on the way to solving the problem.”
ON-LINE HPLC ANALYSIS AND DATA INTEGRATION WITH NUMERA AND LUCULLUS SHOWN ON THE EXAMPLE OF A CHO PROCESS
April 9, 2019HPLC is the common reference method for product and metabolite analysis in bio-processes. Sampling is needed for these analyses, resulting in off-line measure-ments along the process.
NUMERA & LUCULLUS – AN INTEGRATED PAT SOLUTION COMBINING AUTOMATED SAMPLING AND PROCESS CONTROL
April 9, 2019This application note presents two unique PAT tools, which can be used separately or together as an integrated PAT solution. The Numera system enables automated sampling, sample preparation, ...
GLUCOSE MONITORING AND CONTROL USING NUMERA, LUCULLUS SOFTWARE, AND CEDEX® BIO HT ANALYZER
April 9, 2019Critical process parameters (CPPs) such as glucose must be monitored and con-trolled in every bioprocess in order to reach the desired product quality in the most efficient way.
AUTOMATED BIOREACTOR SAMPLING FOR ON-LINE ANALYSIS OF AMINO ACIDS USING TWO APPROACHES: HPLC AND CEDEX® BIO HT ANALYZER
April 9, 2019To facilitate optimal process control limiting substrates must be identified, monitored and controlled. Amino acids are often critical substances, especially in mammalian processes.
Stay on Top of Securecell's Newest Advancements
Subscribe to the Securecell Newsletter and stay up to date with all the latest product news, events and announcements. Don't miss out on any upcoming releases and conferences.